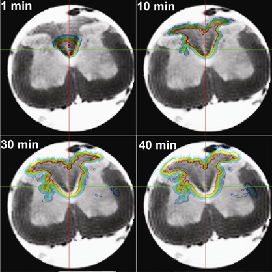
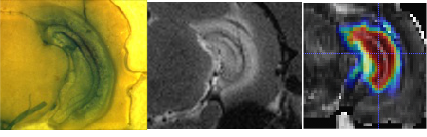
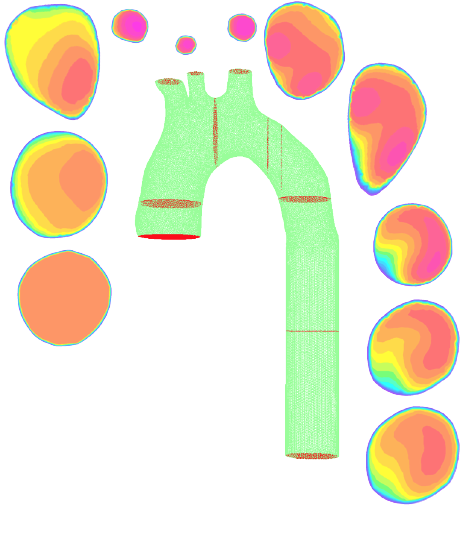
Medical Device and Bioengineering
Reliability and Quality Assurance for Medical Device Design
Reliability and quality assurance for medical device design examines whether medical devices are fit for continued service until the next shutdown based on protection against failure modes (plastic collapse, local failure, buckling and cyclic loading).
Equipment may contain flaws, not meet current design standards or be subjected to more severe operation condition than current design.
Medical device services include
- FEA based fracture assessment
- Heat transfer and thermal stress assessment
- Vibration and fatigue assessment
- Expected lifetime analysis
- Long-term reliability assessment
- Non-linear analysis
- Overall performance evaluation
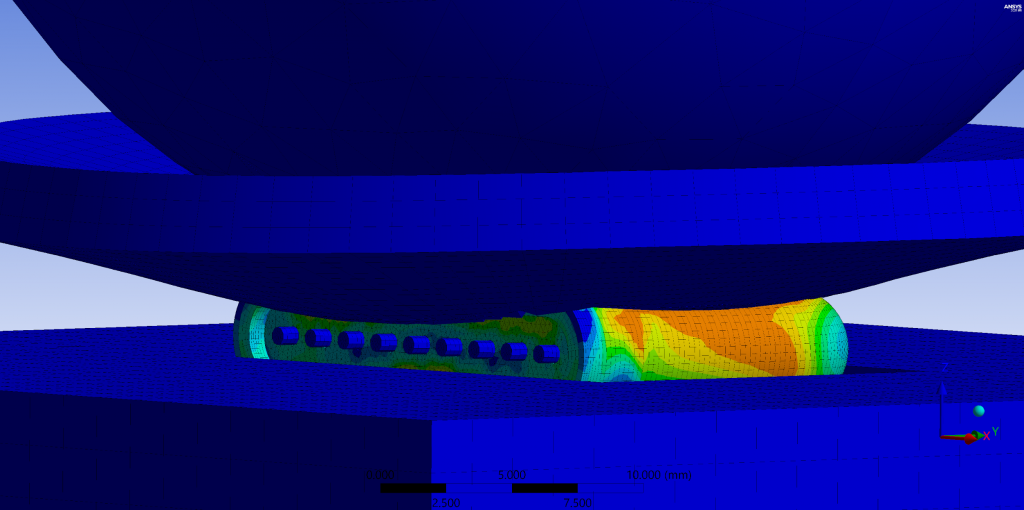
- Miniaturization
- Cost-saving design
- Reliability-improvement design
- Implantable medical device design
- MR-safe implantable device design
- Consultation for development of next generation device
Examples
- Wearable medical device
- Implemental medical device
Bioengineering
Bioengineering covers broad range of biomedical engineering fields including biomedical device design, cardiovascular modeling and patient-specific computational modeling using multi-modal imaging techniques.
Services include
- Patient-specific imaging based
- cardiovascular flow analysis
- Drug delivery analysis
- Blood pump optimization
- Catheter flow analysis
- Spinal implant analysis
- Thermal analysis of human skin
- Medical image-based 3D reconstruction